
The next phase of the INTERGROWTH-21st Project, known as INTERPRACTICE-21st, has now begun.
INTERPRACTICE-21st aims to promote optimal postnatal growth of preterm infants until 6 months post-term by:
(a) standardising growth measurement in babies born preterm by using the INTERGROWTH-21st international standards
(b) implementing evidence-based feeding recommendations based on breast feeding.
We have partnered with leading neonatal units across the world to introduce these interventions in their hospitals and countries, with the ultimate aim of wider dissemination and high-level health policy support. Already 310 institutions/units/individuals have reported using INTERGROWTH-21st Preterm Postnatal Standarts and Feeding Protocols all over the world, as seen on the map below:
Using recommended feeding practices and international standards to monitor postnatal growth and development should help avoid nutritional patterns that may be associated with childhood overweight and obesity, and improve the growth, development and survival of preterm infants.
Click here to access the study protocol and study documents for INTERPRACTICE-21st
To join the Project Complete a simple, descriptive online registration form here:
To participate actively in the Project promote the free e-learning course to be taken by all health professionals providing care to preterm infants in your network or related institutions. All participants will receive a certificate from the University of Oxford/Geneva Foundation for Medical Education and Research on successful completion of the course.
Implement the INTERGROWTH-21st Preterm Postnatal Growth Standards in your healthcare institution. The standards are suitable for monitoring the growth of preterm infants during hospital stay and after discharge up to 64 weeks’ postmenstrual age.
a) Promote the INTERGROWTH-21st evidence-based Feeding Recommendations that are conceptually linked to the standards.
b) Collect data on the status at hospital discharge and feeding practices of all preterm babies cared for at the participating institution. The methods of data collection are as follows:
i. Collect data using a summary Pregnancy and Delivery form. Enter the data into the Project database using the online data management system. Data will be the property of each individual institution. Aggregated data analysis will be conducted using only the variables that are agreed among the investigators.
ii. Institutions are asked that, after hospital discharge, growth of the preterm infant is monitored using the Preterm Postnatal Standards (copies can be downloaded from the website; alternatively, measures can be plotted directly on the chart online).
Neonatal units will need to nominate a local contact person to provide information on programme uptake. Technical guidance and implementation support will be provided through regional offices and the central Coordinating Unit in Oxford.
Contact us for more information: INTERPRACTICE21@obs-gyn.ox.ac.uk
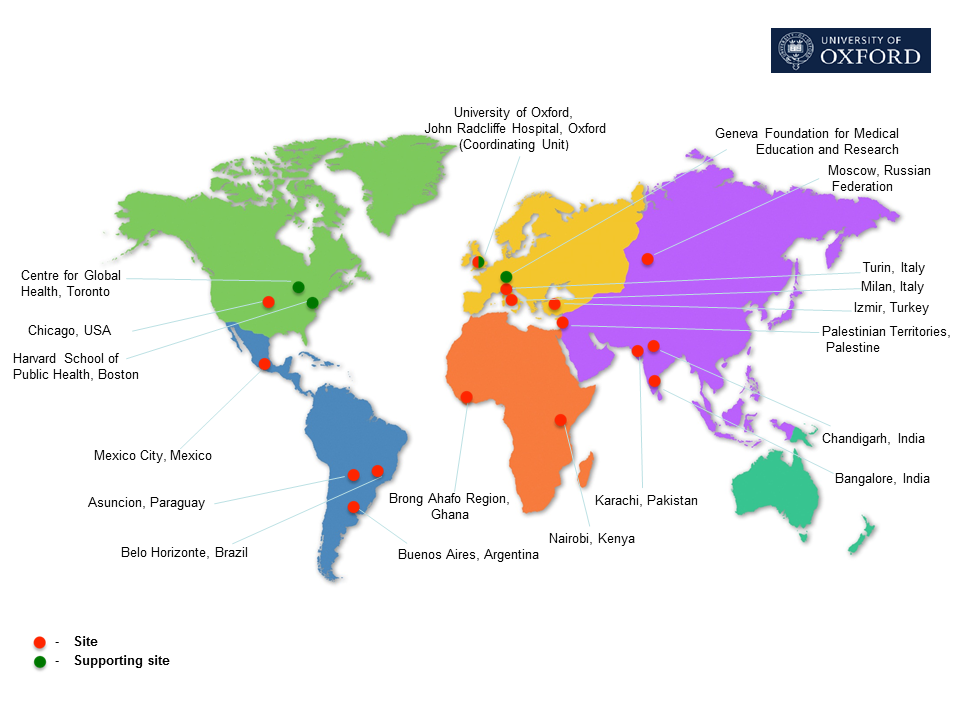 Collaborators in the INTERPRACTICE Project
Collaborators in the INTERPRACTICE Project
Jose Villara, MD, Francesca Giulianib, MD, Fernando Barrosc,d, MD, Paola Roggeroe, MD, Irma Alejandra Coronado Zarcof, MD, Maria Albertina S. Regog, MD, Roseline Ochiengh, MD, Maria Lorella Giannie, MD, Suman Raoi, MD, Ann Lamberta, PhD, Irina Ryuminaj, MD, Carl Brittok, MD, Deepak Chawlal, MD, Leila Cheikh Ismaila, PhD, Syed Rehan Alim, MD, Jane Hirsta, MD, Jagjit Singh Tejin, MD, Karim Abawio, MD, Jacqueline Asibeyp, MD, Josephine Agyeman-Duaho, MSc, Kenny McCormickq, MD, Enrico Bertinor, MD, Aris T Papageorghioua, MD, Josep Figueras-Aloys, MD, Zulfiqar Bhuttat PhD and Stephen Kennedya MD, Ana Langeru MD.
Affiliations
a Nuffield Department of Obstetrics & Gynaecology and Oxford Maternal & Perinatal Health Institute, Green Templeton College, University of Oxford, Oxford, UK
bAzienda Ospedaliera OIRM Sant’Anna Citta della Salute e della Scienza di Torino, Italy
c Programa de Pós-Graduação em Saúde e Comportamento, Universidade Católica de Pelotas, Pelotas, Brazil
d Programa de Pós-Graduação em Epidemiologia, Universidade Federal de Pelotas, Pelotas, Brazil
e NICU, Fondazione I.R.C.C.S. "Ca' Granda" Milano, Italy
f Instituto Nacional de Perinatología "Isidro Espinosa de los Reyes", Mexico City, Mexico
g Departmento de Pediatria, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
h The Aga Khan Hospital, Nairobi, Kenya
i St. John's Medical College Hospital, Bangalore, India
j Center for Obstetrics, Gynecology and Perinatology, Moscow, Russian Federation
k Department of Paediatrics, University of Oxford, Oxford, UK
l Government Medical College, Chandigarh, India
m The Aga Khan Hospital, Karachi, Pakistan
n Ann & Robert H. Lurie Children’s Hospital of Chicago and Mercy Hospital and Medical Center, Chicago, Illinois, USA
o Geneva Foundation for Medical Education and Research, Geneva, Switzerland
p Holy Family Hospital, Techiman, Brong Ahafo Region, Ghana
q John Radcliffe Hospital, Headington, Oxford, UK
r Dipartimento di Scienze Pediatriche e dell’Adolescenza, Cattedra di Neonatologia, Università degli Studi di Torino, Torino, Italy
s University of Barcelona, Barcelona, Spain
t Center for Global Child Health, Hospital for Sick Children, Toronto, Canada
uHarvard School of Public Health, Boston, MA,USA
